CONTENTS
Order from Disorder
Jackson Pollock's Summertime
and the
Emergence of Life
Jackson Pollock painted Summertime, which I saw at the Royal Academy in 2016, in 1948. It is 84cm or 33in high, and 5.5m or 18ft long, an aspect ratio unconducive to small-scale reproduction: it is squashed to a squint, leaving little discernible detail. I therefore reproduce only a moiety; see below.
At first blush, it would appear that Pollock just stood back and chucked paint fecklessly; but this is not the case. He painted with what is called his 'drip' technique. Strictly speaking this is a misnomer, as dripping involves discrete droplets. Rather, he poured direct from the can, allowing paint to fall in unbroken filaments onto the canvas as it lay on the floor. Also, he sometimes directed the flow of paint with a stick, rather than leave its course to gravity alone. This technique resulted in dribbles and squiggles, rather than splats and splashes. Summertime is, then, a carefully constructed work.
What are we to make of it? Some critics, drawing on its unusual aspect ratio, have suggested a musical score: arpeggios, clefs, glissandos and quavers, etc. Schönberg's Suite, Op. 29, for the unusual combination of two clarinets, bass clarinet, violin, viola, cello and piano, comes to my mind. When I described Suite as a cacophony, a musical friend of mine was indignant. 'There are melodies in it - continually shifting melodies!' Not uncoincidentally, perhaps, Arnold Schönberg was an expressionist composer, whereas Jackson Pollock was an expressionist artist. (Cue for another essay, although I'm sure it's been written already). Even so, when I look at Summertime, Rachmaninov does not leap readily to mind. And while we're musically musing, George Gershwin composed Summertime, his aria for Porgy and Bess, in 1935 - perhaps there is some connection? No matter: the painting's title Summertime, I argue here, is surely misconceived.
Other critics have suggested a series of dancing figures, with associated rhythm and movement. The rhythmical property of Summertime I found intriguing because, glanced at perfunctorily, one sees only a formless, amorphous mess. Amorphousness and randomicity might describe other works by Pollock; Convergence, perhaps. Summertime, though, has something different about it; there is a sense of progression, say, through time or space, as one studies it from left to right. There is a central band of black and grey lines; but some cordoned-off areas, filled by patches of yellow, blue and burgundy, appear at regular intervals. Indeed, a definite sense of order emerges from disorder. And this would be my choice of title: Order from Disorder. Rather than music and dancing, therefore, I propose another interpretation of this work; one from the viewpoint of the scientist.
Order from disorder is a widespread natural phenomenon which the general public accept without realising just how weird it is. In the air around us the gas molecules normally whiz about in all directions: this is an incomprehensible anarchy, a disco in which all dancers jiggle away independently, strutting their own funky stuff. Yet, turn on the weather report, and we're shown something very strange indeed: enormous masses of air rotate in a depression or a hurricane or a tornado. Inconceivably large numbers of molecules now participate in a highly organised large-scale structure; a waltz in which all dancers rotate in synchrony around the ballroom. Now, when humans come together in large numbers, they must make agreements. We have committees, local authorities, national governments, treaties, laws, international protocols and many other things. But gas molecules - nonsentient, nonliving particles - require none of these. How on earth do these ordered, coherent structures arise in nature? Have mindless molecules somehow colluded? Well, the first requirement is an energy gradient.
If a fluid is sandwiched between two surfaces, one hotter than the other, thus creating a thermal gradient, then, in certain circumstances, something rather curious happens: as sketched below, there are
cycling convection currents - these are known as Bérnard cells. Fluid heats up at the hot surface, rises, cools at the cold surface, then falls. The view from above, which I also provide, can show some remarkable structures, just one of which is pentagonal. Now obviously we can argue that colder pockets are more dense, and so fall, displacing hotter pockets upwards. But we also know that fluids are composed of molecules; and that molecules are individual actors. Yet, without any traffic lights or even roads, they somehow organise themselves into these elaborate, orderly structures: the molecular motions are no longer anarchical, but seemingly cooperative. As it happens, if the conditions are not quite right, there is a free-for-all in which upward-moving molecules battle against downward-moving molecules. If there are two crowds of people, one of them moving from left to right, the other from right to left, we know how stupid it would be to have a free-for-all: we separate the people into two streams. When we do this, everyone moves so much more quickly. And when Bérnard cells form, the transfer of energy (from hot to cold) is so much the faster. This is an important clue. We are often told that 'nature abhors a vacuum'. A far less-cited maxim is this: 'nature abhors an energy gradient'. Whenever there's an energy gradient, nature will form some ordered structure that degrades or mitigates or dissipates that gradient.
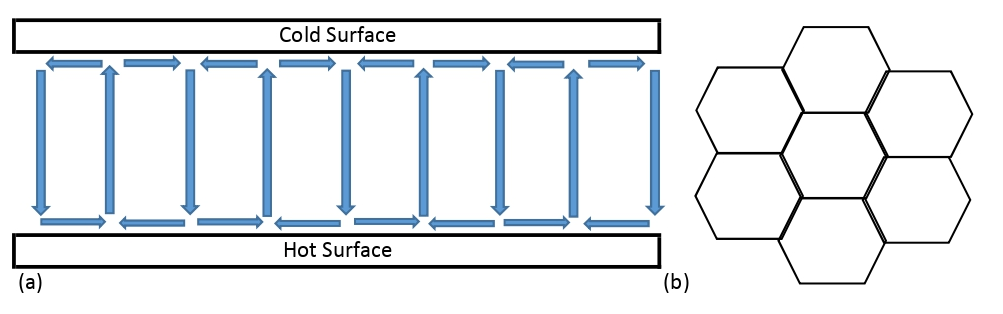
Cyclic convection currents called Bérnard cells form between two plates, one hot, one cold: (a) side view; (b) top view.
Can we quantify 'order'? Yes; scientists speak of entropy, a pivotal concept that emerges from the Second Law of Thermodynamics, and which is, in fact, a measureable physical quantity - it is not at all hazy or nebulous. Now as a general rule, entropy may only increase: that is, disorder may only increase at the expense of order. The total entropy in the universe only ever increases. In fact the universe is evolving toward a state of maximum entropy - this is the so-called 'heat death'. But entropy need not increase everywhere: it can decrease in certain places; provided the total entropy increases. When there are ordered structures of low entropy, such as Bérnard cells, the total entropy is higher than it would be, without them. And these low-entropic structures are entirely self-organising. Even simple physical systems can generate complex, self-sustained structures - these appear spontaneously in nature, where there are energy gradients that can sustain them.
Living organisms are also, in effect, self-organising 'islands' of low entropy. The bodies of plants and animals are highly ordered structures which, by exchanging matter and energy with their surroundings, are able to self-synthesise. But it is entropy that matters, fundamentally. That is to say, an organism maintains itself by importing order from, and exporting disorder to, its surroundings. This is what life is - in the thermodynamic sense, that is. The energy gradient driving all, is that between the daily influx of solar energy, and the earth's re-radiated energy - the same gradient that the biosphere dissipates.
Without this understanding it is impossible to believe that nucleic acids, and numerous other macromolecules necessary for life, arose purely by chance from the primeval soup; not in anything like the length of time the earth has existed: this is statistically so unlikely, that we can rule it out. But, if we understand how order can emerge from disorder, the formation of prolife molecules is no longer improbable. Life probably emerged in response to thermodynamic imperatives, in which energy gradients generated coherent, ordered structures of low entropy, much as a hurricanes generate ordered structures in air; for gradient-based thermodynamics supplies the nexus between nonlife and life. It might be, indeed, that when energy gradients exist, the emergence of life becomes thermodynamically inevitable.
It is for these reasons that Summertime, in which order appears to be emerging from disorder, may be read as a portrayal of the emergence of life.
Bibliography
A.J. Sangster, Warming to Ecocide - A Thermodynamic Diagnosis. Springer (2011).
Dorion Sagan, Jessica Hope Whitesnade (2004). 'Gradient reduction theory: thermodynamics and the purpose of life'. In Scientists Debate Gaia: The Next Century (pp.173-186). MIT Press.
Eric D. Schneider, James J. Kay (1992). 'Life as a manifestation of the second law of thermodynamics'. Mathematical and Computer Modelling, 19(6-8):25-48.
Eric D. Schneider, Dorion Sagan (2005). Into the cool - energy flow, thermodynamics and life. University of Chicago Press.
Erwin Shrödinger (1945). What is Life? The Physical Aspect of the Living Cell. Cambridge University Press.
Harold J. Morowitz (1979). Energy Flow in Biology. Ox Bow Press.
Stuart Kauffman (2000). Investigations. Oxford University Press.
(c) Cufwulf
Cufwulf@aol.com
